SAFETY NOTICE
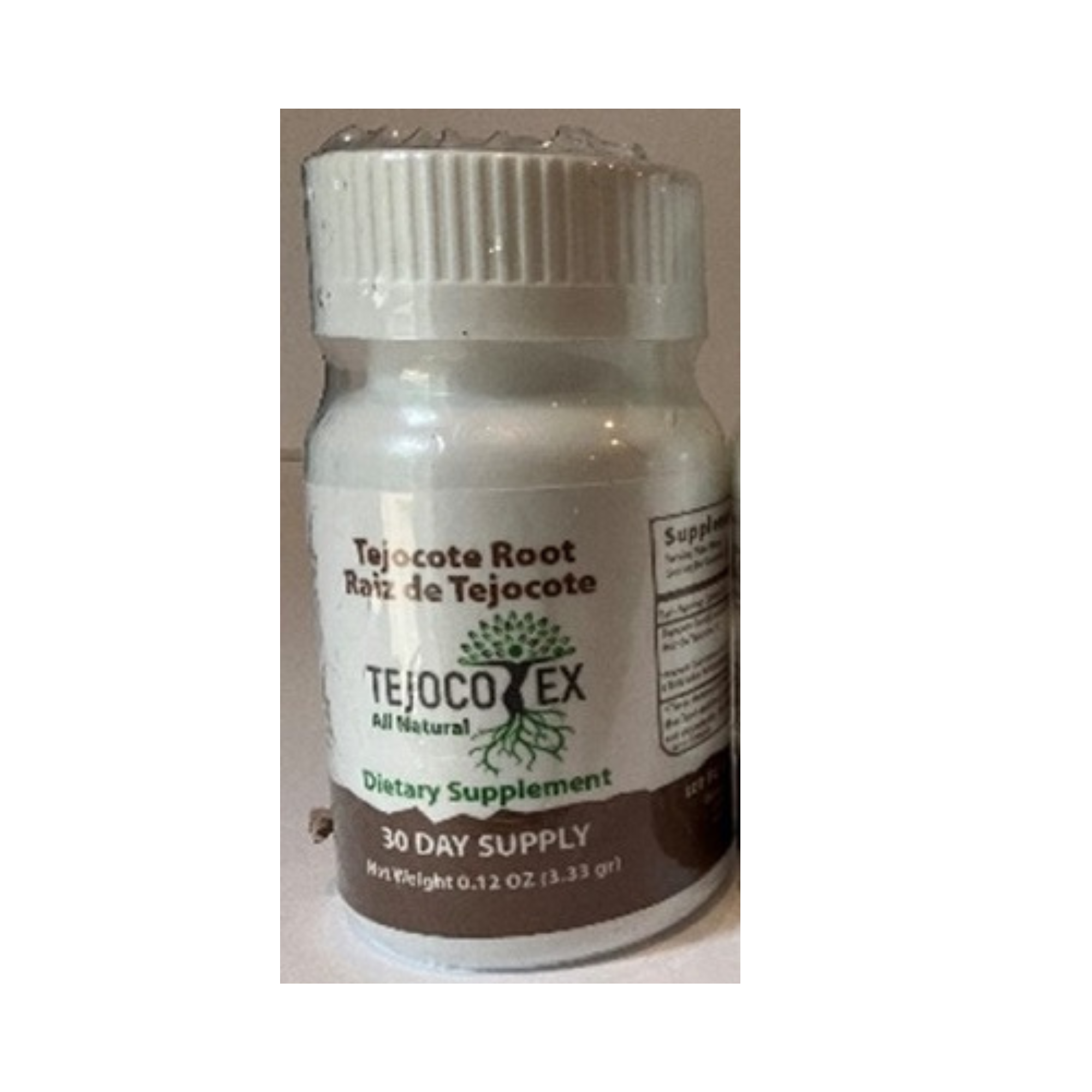
The Food and Drug Administration is advising consumers not to purchase or use Tejocote Root Products (including ELV Alipotec and Tejocotex) as FDA analysis has determined that certain dietary supplements labeled as tejocote (Crataegus mexicana) root were tested and found to be substituted with yellow oleander (Cascabela thevetia).
Recommendation for Consumers
The FDA is advising consumers to stop using these products.
Contact your healthcare provider if you or someone in your care recently ingested these products and have health concerns.
Please contact us by email at contact@innovacionnatural.com or by phone / text at 877-592-0029 if you have questions or need assistance in returning products or inquiring about a refund.


To read full recommendations please visit the Safety notice page
https://www.fda.gov/food/alerts-advisories-safety-information/fda-issues-warning-about-certain-tejocote-root-supplements-substituted-toxic-yellow-oleander
